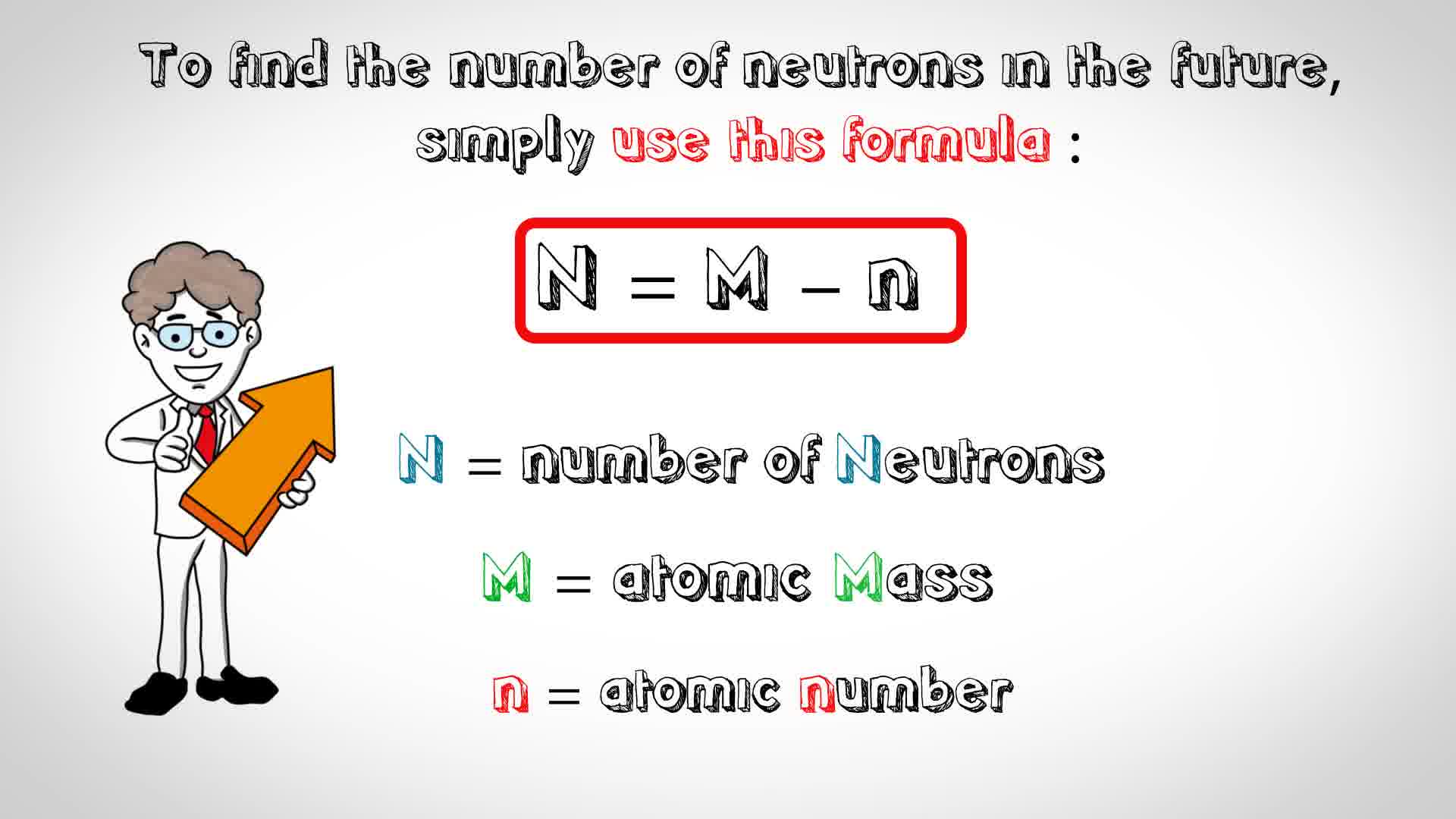Smart Info About How To Find Out How Many Neutrons Are In An Atom

How do you find out how many neutrons a atom.
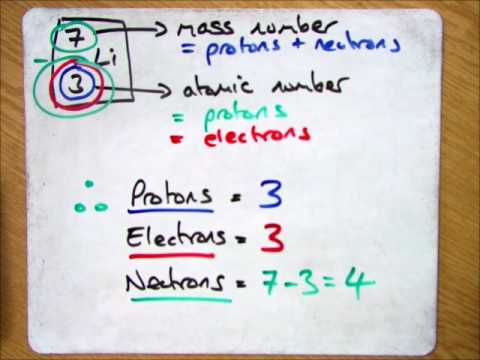
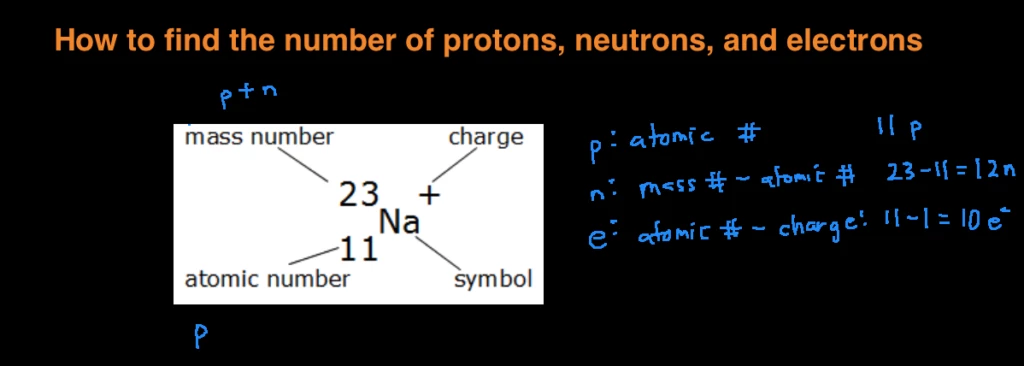
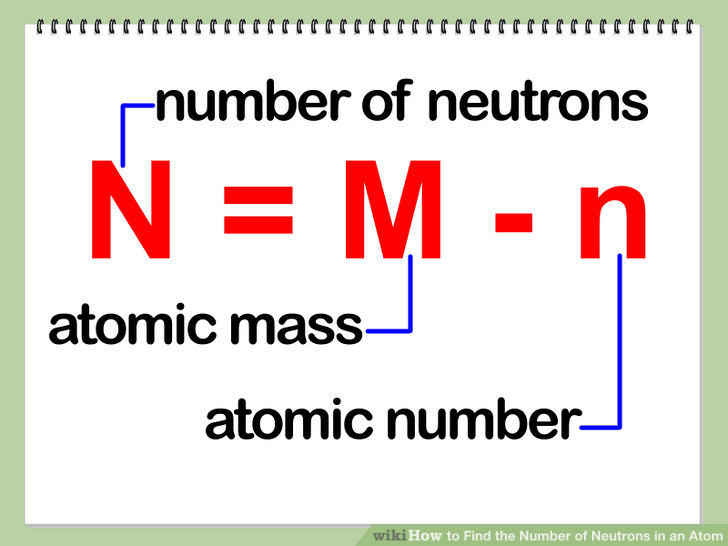
How to find out how many neutrons are in an atom. We do know how many. The number of neutrons in an atom determines its isotope. That is, neutron number (n) = atomic mass.
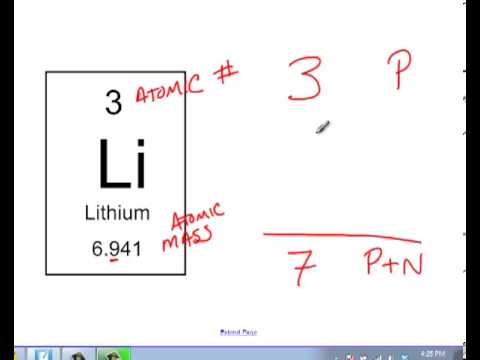
Calculating the number of neutrons then becomes atomic mass of the isotope minus the atomic number of. The difference between the mass number of the iron atom. The number of neutrons in an atom can be determined by the difference between the atomic mass and the number of protons.
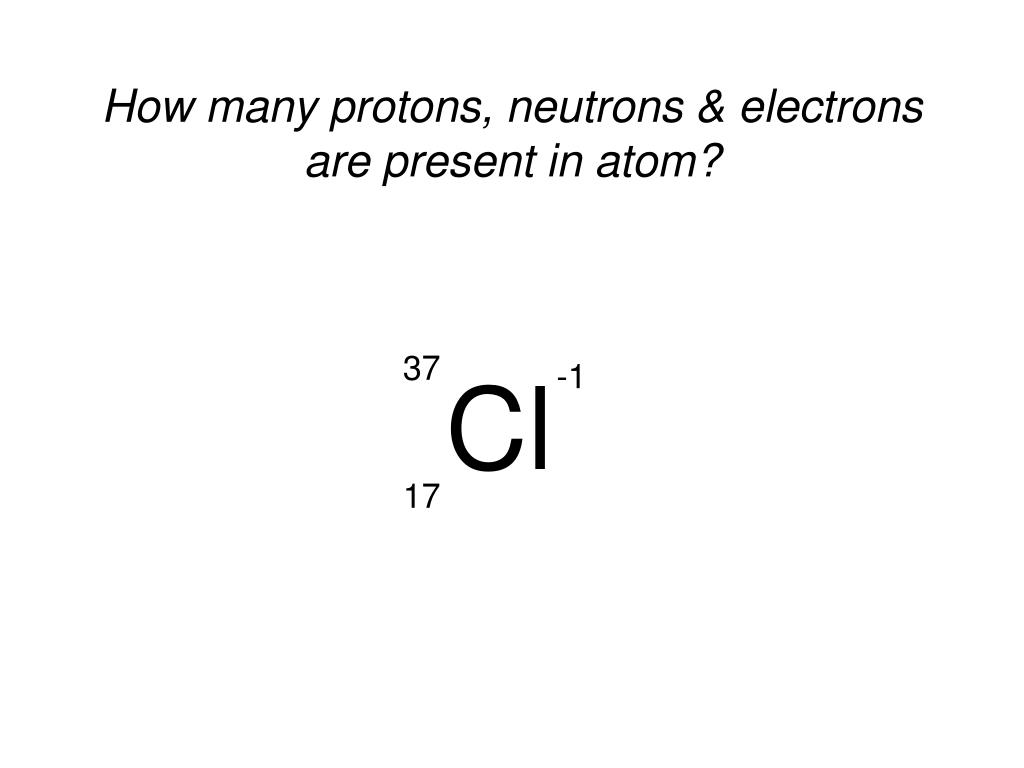
The difference between the mass number of the chlorine. The difference between the mass number of the aluminum. The difference between the mass number of the sodium.
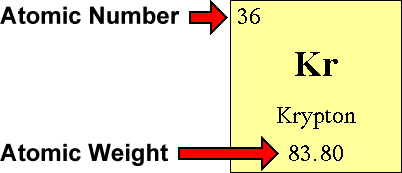
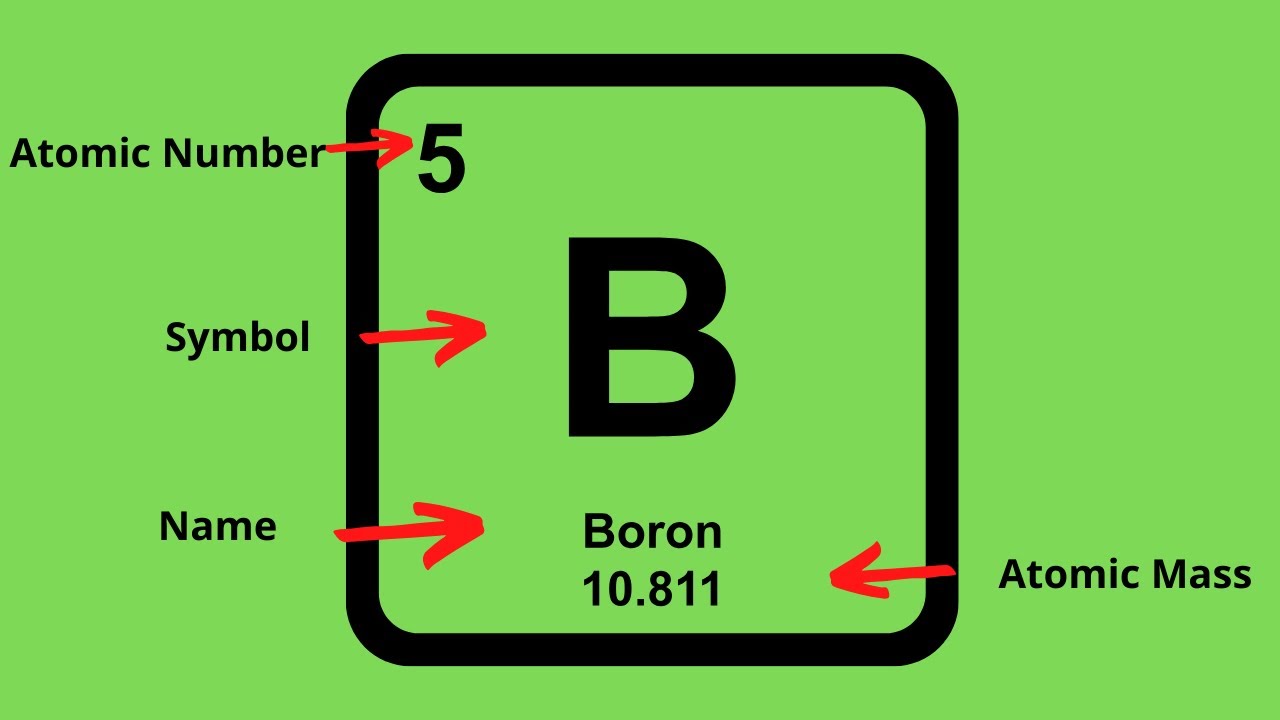
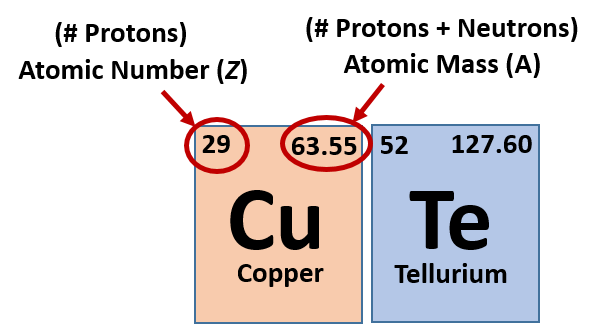
The atomic number of the element equals the number of protons. All you have to do is find the element's atomic number and atomic weight. How to find electrons when an atom is ionized, it loses one or.
Subtract the atomic number from the mass number to find out the number of neutrons. In actuality, all elements are found in nature with varying numbers of neutrons in their. Finding out how many neutrons are in a atom is easy.
The number of neutrons in an atom can be determined by the difference between the atomic mass and the number of protons. The number of neutrons in an atom can be figured by subtracting the atomic number from the mass number. We know that mass number is the sum of protons and neutrons in the nucleus.